 |
Sozai/Jiten/Datacraft/Getty Images |
|
The changing development paradigm resulting from FDA’s quality-by-design (QbD) initiative and International Conference on Harmonization (ICH) guidelines requires increased process understanding of the drug substance and drug product throughout development and manufacturing. A lack of information can result in delays in regulatory approval and higher costs. Applying QbD principles leads to greater process understanding, facilitates regulatory approval, and streamlines postapproval changes. Case studies on the manufacture of a bulk powder and the development of a tablet show the application of QbD principles, including defining critical quality attributes, implementing risk assessment, optimizing process development, developing a design space, and performing a criticality analysis.
Quality by design (QbD) is often misquoted, misused, and misunderstood. Pharmaceutical QbD is a systematic scientific risk-based approach to pharmaceutical development that begins with predefined objectives that address product and process understanding and process control (1). Many articles focus on what is required with respect to product quality, safety, and efficacy but successful approaches are not commonly shared. Successful product development relies on consistent application of a proven methodology. The key steps are the same, irrespective of the product or formulation being developed. A proven methodology is described in this article, with the framework shown in Figure 1. These main steps are further described as outlined below.
Main steps of a QbD process
Critical quality attributes (CQAs). CQAs are defined based on the target drug profile. These are quality characteristics of the drug that must be kept within appropriate limits to ensure the desired product quality (e.g., purity, crystalline form, and particle size).
Risk assessment during the development phase. For each CQA, an analysis of the potential critical process parameters (pCPPs) and potential critical material attributes (pCMAs) is conducted. The aim is to evaluate in each process step, operating parameters or raw materials that have the potential to affect a CQA within the known ranges, and therefore, should be monitored or controlled to ensure the desired quality. Because the number of parameters is usually high, a risk assessment based on prior knowledge of the product or process is used to rank the parameters in terms of perceived criticality. The ultimate goal is to keep the development process as lean as possible by focusing the studies on those parameters with a higher likelihood of having a critical impact.
Process development. The output of the risk assessment is a qualitative match between CQAs and pCPPs/pCMAs. To confirm the dependences and quantify the effects, a process-development stage is conducted. Usually a statistical approach is followed, through a sequence of design of experiments with different objectives--screening, optimization, and robustness studies. This development stage constitutes the core of the QbD methodology since most of the process knowledge is generated during this stage. Although not mandatory, a model, either statistical and/or mechanistic, is a usual outcome of this stage. Process analytical tools can also be considered at this stage based on the need to improve the CQA monitoring as the process is scaled up.
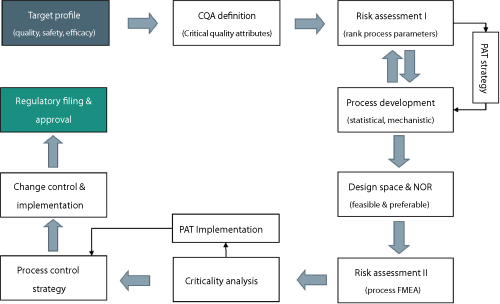 |
| Figure 1: An overview of Hovione’s quality-by-design approach. CQA is critical quality attribute, PAT is process analytical technology, NOR is normal operating range, FMEA is failure mode effect analysis. All Figures are courtesy of the authors.
|
|
Design space and normal operating ranges (NOR). Once the impacts of the pCPPs/pCMAs are quantified on the CQAs, a feasible operating space can be defined. This space, known as the design space, will consider all the interactions between operating parameters and material attributes and will often be multidimensional. The NOR is established within the design space, and can be thought of as the ranges where the process typically operates.
Risk assessment during manufacturing. After defining the design space and NOR, an exhaustive analysis of the process is conducted at the manufacturing scale. In this study, a failure mode effect analysis (FMEA) of all manufacturing aspects are reviewed, challenging the equipment operating ranges and procedures against the process knowledge gathered in the previous steps. The purpose of this study is to understand and quantify the risk of batch or process failure and to define actions to minimize failures.
Criticality analysis. By knowing the feasible operating regions and after evaluating the equipment/procedures at the manufacturing scale and the practical NOR, a final criticality analysis will take place to identify parameters and/or material attributes that will require tight monitoring or control. For example, all parameters for which the corresponding NORs are close to the boundaries of the design space.
Process-control strategy. Once the criticality around a process parameter and/or raw material attribute is confirmed, adequate control strategies will be set in place. The ultimate goal is to assure that the operation is always taking place within the design space, therefore, assuring the quality of the final product. For this purpose, and considering the dependence of a control strategy on a given monitoring capability, the final implementation of process analytical technology tools is carried out at this stage. The subsequent steps are mainly focused on the documentation aspects associated with the filing process and will not be addressed in this article.
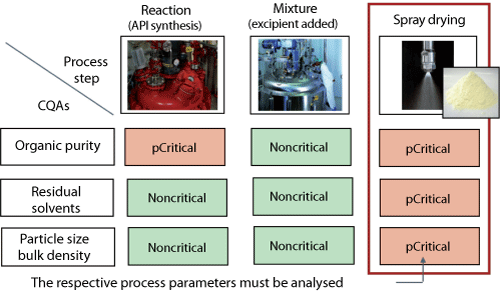 |
| Figure 2: Risk assessment. Decomposing the process in main steps for a more structured criticality assessment (illustrative example for the bulk powder manufacturing process). CQA is critical quality attribute, p is potential. |
|
Bulk powder development case study
CQA definition. This case study examines the preparation of bulk powder that is subsequently formulated as a tablet. The preparation of the powder was broken down into three stages: synthesis, excipient addition, and spray drying. The spray-drying stage was identified as being potentially crucial for all CQAs, and will be examined in more detail (see Figure 2). CQAs for the bulk powder were determined to be purity, residual solvent level, particle size distribution, and bulk density among others, but will not be addressed in this article.
Risk assessment. A risk assessment was completed to prioritize and reduce the number of parameters to be investigated in the study. This process is subjective and relies on the experience of the team members involved in the assessment. Having four or more inputs will help reduce bias and enable the top pCPPs to become evident in general (see Figure 3). It is important to recognize that at this point, all process parameters are only potentially critical; confirmation of criticality is only conducted later in the methodology.
Although identified as being a pCPP, certain parameters may need to be fixed because they impact other aspects of the process such as yield and throughput. In this study, the concentration of the feed solution was fixed and the outlet temperature (T_out), the feed pressure (P_feed), and the spraying nozzle diameter (D_noz) were varied.
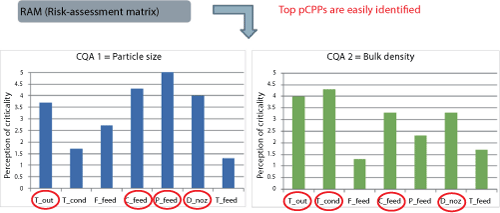 |
| Figure 3: Risk assessment: ranking of potentially critical process parameters per critical quality attribute (CQA) in each process step as the output of a risk-assessment matrix (bulk powder manufacturing process). T_out is drying gas temperature at the outlet of the spray drying chamber (ºC); T_cond is drying gas temperature at the exit of the condenser (ºC); P_feed is atomization pressure of the feed (pressure nozzle) (bar); D_noz is diameter of the nozzle orifice (mm); T_feed is temperature of the solution fed to the spray drier (ºC), F_feed is flow rate of feed solution (kg/h); and C_feed is concentration of feed solution (% w/w). |
|
A series of experiments were run as a screening study. Using a statistically valid design of experiments (DOE), eleven runs were made. These trials considered a 24-1 half-factorial design with the centre point run in triplicates (see Figure 4). Once complete, the ranges of a DOE become the knowledge space for your product. Subsequent studies enlarge the knowledge space.
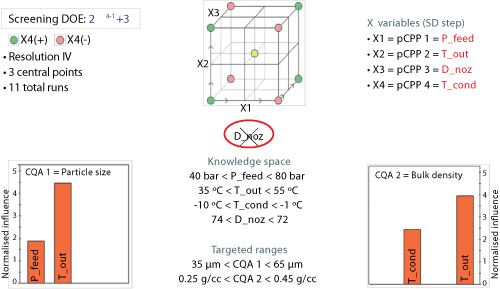 |
| Figure 4: Design of experiments (DOE) (screening phase). Confirming the risk-assessment output by checking statistical significance of the most ranked parameters (bulk powder manufacturing process). CQA is critical quality attribute; SD is spray drying; pCPP is potentially critical process parameter; P_feed is atomization pressure of the feed (pressure nozzle); Tout is drying gas temperature at the outlet of the spray drying chamber; D_noz is diameter of the nozzle orifice (mm); T_cond is drying gas temperature at the exit of the condenser. |
|
Data from this study indicated that a large portion of the knowledge space is viable to produce acceptable product. Subsequently, an optimization DOE was run. Study resolution was enhanced with the addition of a third level at the midpoint. With two center point runs, this second study required 16 runs. Data from this study led to a refinement of the model and the generation of quadratic terms to describe the particle-size relationship (see Figure 5).
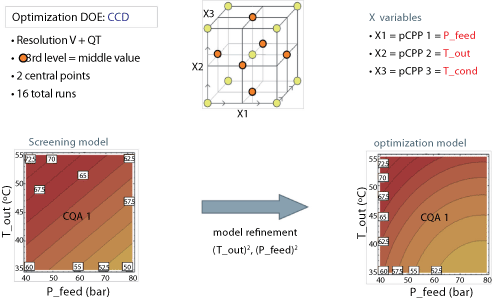 |
| Figure 5: Design of experiments (DOE) (optimization phase). Increasing the prediction accuracy of screening models (for design space establishment) via refinement of the mathematical relationships (bulk powder manufacturing process). CCD is central composite design; CQA is critical quality attribute; T_out is drying gas temperature at the outlet of the spray drying chamber; P_feed is atomization pressure of the feed (pressure nozzle). |
|
At this stage, models exist for each CQA. As both CQAs must be met simultaneously, the design space will narrow, adding complexity to the problem. One further level of complexity comes from the uncertainty in the model. Working at the edge of a modeled range brings risk to the process. Working, for example, at the 95% confidence interval of the model reduces the risk of generating material with CQAs outside the specification limits while maintaining a broad operating space (see Figure 6).
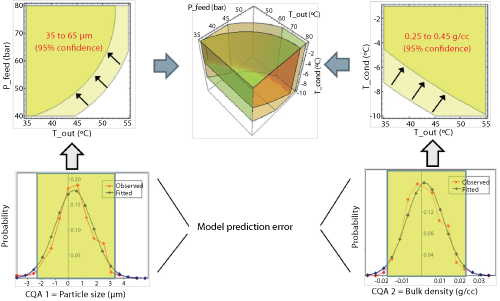 |
| Figure 6: Uncertainty analysis. Considering model prediction errors to regress the boundaries of the design space and, in this way, define confidence levels for the resulting operating spaces. CQA is critical quality attribute; P_feed is atomization pressure of the feed (pressure nozzle); T_cond is drying gas temperature at the exit of the condenser; T_out is drying gas temperature at the outlet of the spray drying chamber. |
|
The NOR is defined as the preferable operating range within the identified design space. Working within this sub-region of the design space, the NOR may have benefits of reduced operating costs and increased productivity or preferential product characteristics. The NOR is dependent on the controllability of the process, which may be equipment or plant dependent. For example, the temperature control of the equipment may be limited to +/- 1.0 °C, thus a NOR tighter than +/- 0.5 °C is not achievable.
A criticality analysis will determine which process parameters need to be most closely monitored. Each process parameter will have a different effect on a CQA. Normalizing these impacts will highlight which parameter exerts the greatest influence on a CQA. Larger normalized values imply increased sensitivity and potentially undesirable effects on CQAs. Ideally, the NOR should be away from the edge of the design space and correspondingly, the design space should be away from the edge of failure. Proximity of the design space towards the edge of failure could mean that deviations from the design space result in out-of-specification material. In this specific example, the achievable NOR in equipment “A” was small relative to the design space and control was readily achieved (see Figure 7).
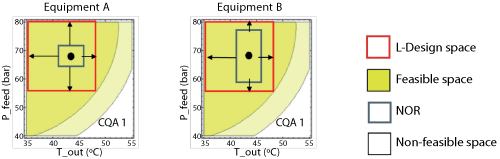 |
| Figure 7: Criticality analysis. Proximity of the normal operating range (NOR) towards the boundaries of the linear-design space. Desirable (Equipment A) and Undesirable (Equipment B) scenarios (bulk powder manufacturing process). CQA is critical quality attribute; P_feed is atomization pressure of the feed (pressure nozzle); T_out is drying gas temperature at the outlet of the spray drying chamber. |
|
Drug product case study
The identical methodology to that used for the bulk powder can be applied to the development of a drug product.
CQA definition. A finished dosage form has a number of CQAs, some under regulatory control and others that are product specific. In this example, a direct compression formulation was considered, where a spray-dried dispersion (SDD) was a significant component of the final formulation. The CQA of tablet hardness will be examinedin greater detail.
Risk assessment. The components of the formulation are the SDD, excipients as compression aids, the disintegrant, and the lubricant. The level of excipient was fixed to limit the resulting tablet size. The risk assessment for tablet hardness determined that the SDD properties, lubricant level and mixing time, tablet press speed, and compression force were pCPPs.
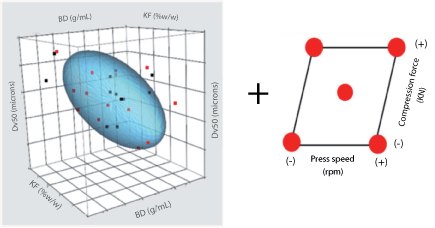 |
| Figure 8: Design of experiments (screening phase). Linking critical quality attributes of the intermediate bulk powder process with potentially critical process parameters of the final dosage form process (tabletting). Dv50 is volumetric mean particle size of the product; KF is the residual moisture of the bulk powder by Karl-Fischer; BD is the bulk density of the product. |
|
Process development. Figure 8 shows the relationships between particle size (Dv50), bulk density (BD), and moisture content (KF) that were determined from a separate series of DOE studies. Additionally, it also shows the range of material properties that could be prepared. For the compression analysis, materials indicated by the red points were selected to give a broad range of physical properties. Blends were prepared and, after some preliminary ranging studies, were run at two press speeds and two compaction forces. The resulting correlation with tablet hardness for the process parameters examined show a weak relationship to KF and BD, and more sensitivity to compaction force and press speed despite studying a relatively low range of press speeds (see Figure 9). From this study, target hardness specifications were generated and will be re-evaluated as the scale-up work progresses.
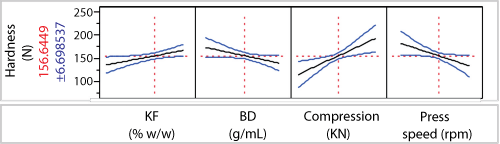 |
| Figure 9: Design of experiments (screening phase). Modeling relationships between critical quality attributes (tablet hardness) and different potentially critical process parameters (final dosage form process). KF is the residual moisture of the bulk powder by Karl-Fischer (% w/w);BD is the bulk density of the product (g/mL). |
|
Conclusion
In summary, QbD is a synonym for process understanding. The greater the understanding of the process, the less likely the generation of out-of-specification material. In the development process, a qualitative risk assessment helps contain the development scope and use a manageable number of experiments to define the design space. The use of statistical design approaches is essential to address an appropriate number of parameters and interactions. Once a model is generated, uncertainty and criticality analysis should be factored in to the definition of the design space to ensure that the operation is not taking place close to the edge of failure, or when it is, that a proper control strategy is set accordingly.
Reference
1. ICH, Q8 (R2) Pharmaceutical Development (2009).
About the Authors
Conrad Winters*, PhD, is director, Drug Product Development Group, cwinters@hovione.com, and Filipe Neves, PhD, is group leader, Drug Product Development Group, both at Hovione FarmaCiencia SA.
*To whom all correspondance should be addressed.









