 Booth 1801
Booth 1801
Capsule Filters
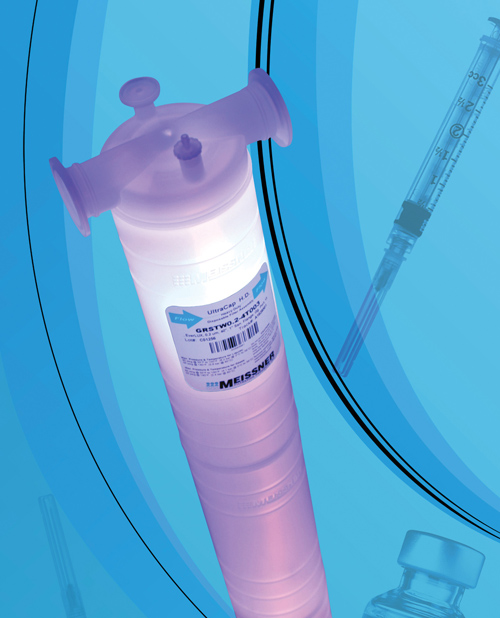
UltraCap HD (heavy duty) single-use high capacity capsule filters provide high flow rates and high throughput, in lengths up to 40 inches for large-volume filtration. The UltraCap HD resists damage caused by rough handling conditions and demanding environments. It has no sharp edges, so it can be integrated into single-use systems. The UltraCap HD is optimized for continuous and batch processing in biomanufacturing operations, and for final and prefiltration in pharmaceutical applications. Additional lengths of 10-, 20- and 30-inches give end users scale-up flexibility. Offered with T-style sanitary flange inlet and outlet connections, UltraCap HD can be specified with a choice of filter media removal ratings from 0.04 μm to 99 μm. UltraCap HD is available gamma-irradiated for aseptic applications. Meissner Filtration Products, Inc., 805.388.9911, www.meissner.com.
 Booth 1649
Booth 1649
Trace Contaminant Removal
The Mobius FlexReady solution for trace contaminant removal features ChromaSorb devices—single-use flow-through membrane adsorbers designed for removing trace impurities including host cell protein (HCP), DNA, endotoxins, and viruses from monoclonal antibody or other protein feedstocks. With Mobius FlexReady Solutions, users can install equipment, configure applications, and validate their processes quickly and easily, shortening development and manufacturing time. The pre-assembled, pretested Flexware assemblies include Millipore technologies such as Millistak+ Pod filters, Pellicon 3 TFF cassettes, Viresolve Pro parvovirus removal filters, Millipore Express sterilizing-grade filters, Lynx sterile connectors, and Mobius single-use mixing and storage systems. The process-ready hardware systems are ergonomically designed for fast setup, maximum adaptability to changing process needs, and minimized operator error risk. EMD Millipore, 800.548.7853, www.millipore.com.
 Booth 2006
Booth 2006
Biotech Filtration System
The PallSep Biotech system for the separation of target molecules from complex biotechnology process fluids system uses vibrating membrane filtration (VMF) technology and encapsulated hydrophilic polyethersulfone membrane filter modules. This combination reduces the accumulation of retained species on the membrane surface, which allows for increased transmission of the target molecule. The VMF principle is based on the rapid oscillation of porous membrane plates in a process feedstream to create shear at the membrane surface. The encapsulated 0.2 µm polyethersulfone membrane filter modules, available in filter areas of 0.2, 1, and 5 m2, offer flexibility for scale-up and scale-down of processes. Pall Corporation, 800.717.7255, www.pall.com/biopharm.
 Booth 1819
Booth 1819
Single-Use Biomanufacturing System
Sartorius Stedim Biotech’s FlexAct enables biomanufacturers to use integrated single-use equipment for all biomanufacturing steps in upstream and downstream processing. As a full-fledged workstation, FlexAct offers new possibilities for designing efficient and flexible production processes by using out-of-the box disposable operations. FlexAct BP is designed for preparation of buffers that are essential for stabilizing target molecules and providing for their optimal production conditions. The FlexAct BP system is based on a multifunctional central operating module, which accommodates preconfigured single-use assemblies, and a pump and a control unit. FlexAct BP addresses the entire development cycle and production capacity needs from 50 to 1,000 L for buffer preparation. Sartorius Stedim Biotech, 800.368.7178, www.sartorius-stedim.com/flexact.
 Booth 2460
Booth 2460
Bioprocessing Software
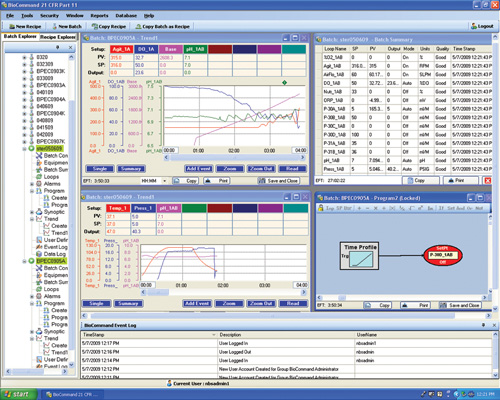
BioCommand Batch Control Plus from New Brunswick Scientific was specifically designed for FDA-validated fermentation and cell culture processes. This software incorporates advanced security features, event logs, and audit trails to help you meet 21 CFR Part 11 guidelines. It enables remote monitoring and control of several fermenters or cell culture bioreactors from a single PC, and is OPC-compatible, so you can integrate data from other OPC-compatible devices into your process. Drag-and-drop icons allow you to create advanced control algorithms, so no programming is needed.
New Brunswick Scientific, 877.723.3319, www.nbsc.com.
 Booth 2909
Booth 2909
Biological Safety Cabinets
Telstar biological safety cabinets were developed for laboratories with limited space. Intended for the laminar flow manipulation of microorganisms with biological risk levels 2 and 3, the Telstar Bio II Advance cabinets offer an ergonomic design that adapts to small spaces, especially in laboratories with low ceilings and where various pieces of equipmentmust be used in the same area. The Bio II Advance is designed to protect the user, the sample, and the environment, and is notable for its reduced external dimensions while maintaining an optimum inner workspace. With a width of 759 mm, the height of the Bio II Advance is up to 20% less than the market average. Telstar, +34 93 736 1600, www.telstar.eu.
 Booth 1801
Booth 1801 Booth 1649
Booth 1649
 Booth 1819
Booth 1819
