 |
| Micah Young/Getty Images |
Concerns for safety in administration of injectable drug products have escalated in recent years, and as a result, increased scrutiny of administration practices including consistent withdrawal volume has occurred. In addition, many drugs administered on a regular basis for chronic conditions are now being offered for at-home preparation and administration. This shift highlights the importance of providing therapies that are not only effective, but also easy and convenient to use by providers other than healthcare professionals.
Delivering a consistent dose of drug product to the patient is a crucial aspect of patient safety and therapeutic efficacy. Excess volume is often added to a vial to compensate for the variability in technique of drug withdrawal or reconstitution using a needle and syringe.
For drugs in short supply, formulating a minimum and adequate overfill is vital to maximize available drug product so that the patient’s therapy is not interrupted. The United States Pharmacopeia (USP) General Chapter <1> Injections provides that each container of an injectable product is filled with a volume that slightly exceeds the content indicated in the labeling (1).
Even when appropriately labeled, however, single- and multi-dose vials that contain significantly more drug than is required for the dose may result in the misuse of the leftover drug product. Similarly, the need to combine several single-dose vials for a single-patient dose may lead to medication errors and microbial contamination.
Inappropriate excess volume and labeled vial fill sizes are two factors that may contribute to unsafe handling and injection practices by consumers and healthcare providers. In March 2014, FDA published
Draft Guidance for Industry, Allowable Excess Volume and Labeled Vial Fill Size in Injectable Drug and Biological Products, which addresses fill and packaging issues for injectable drug products packaged in vials and ampules. The draft guidance suggests that single-dose vials should not contain a significant volume beyond what would be considered a usual or maximum dose for the expected use of the drug product (2).
Innovations in the drug delivery market have provided administration systems such as needle-free transfer devices, including vial adapters, which are designed to provide consistency when transferring liquid for reconstitution of lyophilized drug products from a vial. An internal study (West Pharmaceutical Services) demonstrated that this consistent withdrawal, by use of a vial adapter, can minimize the impact of user variability and has the potential to aid in a reduction of the overfill associated with withdraw variability across users.
Misuse can lead to patient risk
According to FDA, unsafe handling and injection techniques have led to vial contamination and an increased risk of bloodborne illness transmission between patients (2). Inappropriate excess volume may contribute to these practices. When there is more drug than is required for a single dose, pooling of leftover doses may result, a practice that can lead to multiple punctures that may compromise vial integrity and increase the potential for contamination. In addition, excesses or deficiencies in volume may lead to medication errors if the withdrawn dose is too high or too small.
Allowable excess volume, or overfill, must follow the requirements of 21 CFR 201.51(g) and comply with
USP General Chapter <1151>, which requires justification for deviations from the recommendations. FDA recommends that:
- Single dose vials should not contain a significant volume beyond what would be considered a usual or maximum dose for the expected use of the drug product
- Multiple dose vials should contain no more than 30 mL of the drug product except under specific circumstances.
Determination of the appropriate vial fill volume and excess volume should be included during formulation development and should happen no later than the end of Phase II testing.
Minimizing variation of withdrawn volume
Maintaining a consistent dose of drug product to the patient is a crucial aspect of patient safety and therapeutic efficacy. Reconstitution of lyophilized drug product has historically occurred through the transfer of liquid from the diluent vial to the lyophilized drug vial through the use of a disposable needle and syringe. This method can create safety concerns and potential needle-stick injuries.
A variety of technologies are available to aid in the reconstitution and administration of drug products, including vial adapters. A vial adapter functions by snapping securely over the top of a vial, while utilizing a plastic spike to puncture the rubber stopper. It is compatible with Luer lock and Luer slip syringes for liquid transfer. The process of reconstitution and transfer of drug product is accomplished without the use of a needle.
Vial adapters allow rapid transfer and reconstitution of drugs between vials and syringes. Vial adapter spike technology provides a reproducible engineered depth for drug and diluent aspiration, which reduces the end-user variability associated with traditional needle aspirations, according to internal company testing. Vial adapters are designed to reduce exposure to needle-stick injuries; provide fewer steps, parts, and sharps versus needle and syringe systems; reduce overfill requirements associated with withdraw variability across users; simplify the transfer and reconstitution process for novice and experienced users; and, reduce the incident of coring associated with hollow needle penetration of the stopper, as demonstrated by West Pharmaceutical Services studies.
Options for vial adapters include air venting, which features a dual-channel spike that facilitates rapid large-volume withdrawal without having to manually over pressure the vial. The design allows for sterile inbound air and proper aspiration of the drug product while reducing sprayback potential of toxic materials through pressure equalization. A swabable vial adapter features a sterile resealable silicone rubber valve with a Luer-compatible connector that helps to maintain sterility for repeat vial access. For multi-dose vials, this adapter can help to reduce the potential for coring and stopper integrity issues. In addition, the swabable valve opens only when connected to/compressed by standard Luer slip or Luer lock syringe to help prevent misuse.
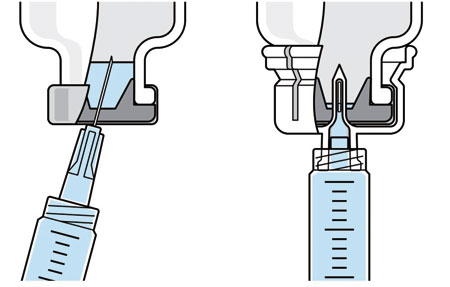 |
| Ordinary drug withdrawal |
With West vial adapter |
| Figure 1: Schematic of needle (left) and vial adapter (right) aspiration methods. |
|
To better understand industry concerns surrounding consistent dosing, a study sponsored by West was initiated to compare the volumes of liquid withdrawn from a vial using two methods: traditional needle penetration and vial adapter with spike penetration. Figure 1 provides a schematic of the two aspiration methods. On the left is a depiction of the needle penetration method using a standard needle and syringe system. On the right is a depiction of the vial adapter, which uses a spike in the center of the device to puncture the rubber stopper.
The study compared the withdrawal capabilities of users with varying skill levels using a vial adapter (see Figure 2) and a traditional syringe and needle system.
 |
| Figure 2: Consistent withdrawal, through the use of a vial adapter can minimize the impact of user variability. |
|
Methods
The study included an evaluation of three groups of users and two aspiration methods. Table I shows the test matrix, which includes one control group and two experimental groups. The experimental groups consisted of 10 certified nurses and 10 hemophilia patients who had not previously used the vial adapter devices. These groups were chosen as representatives of the clinical (nurses) and at-home (hemophilia patients) administration settings. The control group consisted of four experienced lab analysts with previous experience using the vial adapters.
The subjects were provided with instructions for use, a video presentation, and verbal instructions on how to aspirate the liquid using the two methods. They were supplied with vials containing distilled water, plastic disposable Luer-lock syringes, 19-gauge needles, and 13-mm vial adapters with siliconized spikes. Each subject group completed 100 aspirations using each method. The volume of aspirated liquid was determined by weighing the syringes before and after the aspiration procedure. Nurses were given a nine-minute time limit to complete all 10 aspirations for each method to more closely simulate their actual working conditions.
Results and conclusion
The average volume withdrawn for each subject group and the standard deviation for all the subjects within that group are provided in Table II. The lab analysts’ (control) results were similar for the needle and vial adapter withdrawal. A more significant difference with withdrawal volume between needle and vial adapter withdrawal methods was noted for the nurse group (experimental). The average volume withdrawn was similar between the two methods, but the standard deviation was greater using the needle. The patient group (experimental) demonstrated the greatest variability between the needle and vial adapter withdrawal methods.
| Table I: Demographic of test subjects. |
Aspiration method |
Patients |
Nurses |
Lab analysts (Control) |
13-mm vial adapters |
10 x 10 times |
10 x 10 times |
4 x 25 times |
Needle (19-gauge) |
10 x 10 times |
10 x 10 times |
4 x 25 times |
Overall, the use of the vial adapter resulted in greater consistency for liquid withdrawal than the use of a traditional needle withdrawal for both experimental groups. To control the volume of drug product delivered to a patient, it is necessary to first confirm that the appropriate volume of liquid is present in the delivery device and that consistency is maintained from dose-to-dose, as well as user-to-user. As demonstrated in this study, the use of the vial adapter is one method for maintaining consistent liquid withdrawal across doses and users.
| Table II: Time to visual cleanliness (min). |
|
Needle |
Vial Adapter |
|
Volume withdrawn (mL) |
Standard deviation (mL) |
Volume withdrawn (mL) |
Standard deviation (mL) |
Lab analysts |
2.04 |
0.102 |
2.04 |
0.025 |
Nurses |
1.98 |
0.106 |
1.99 |
0.057 |
Patients |
1.92 |
0.237 |
2.00 |
0.047 |
Because the vial adapter can provide more consistent dosing withdrawal, its usage may also provide the opportunity to reduce overfill associated with variability of dosing across users. Reducing risk of inadequate or excessive overfill protects both the patient and the pharmaceutical manufacturer. For a patient or a nurse, the use of the vial adapter provides confidence that they are providing a consistent drug withdrawal. A pharmaceutical manufacturer may realize benefits of cost controls associated with overfill practices and a contribution to patient safety as a result of facilitating proper dosing.
It is recommended that pharmaceutical manufacturers determine the optimum amount of withdrawal volume of the proposed formulation for intended use early in the development process. Manufacturers should then test the residual liquid left in the vial at several overfill volumes, such as 5, 10, 15, and 20% when using the appropriate vial adapter to determine minimum excess volume. By including technologies such as vial adapters during the clinical studies, pharmaceutical manufacturers will be able to evaluate and potentially reduce the excess volume associated with withdraw variability across users.
References
1. USP 34-NF 29 General Chapter <1>, “Injections”.
2. FDA, Draft Guidance for industry Allowable Excess Volume and Labeled Vial Fill Size in Injectable Drug and Biological Products (Rockville, MD, 2014).
About the author
Zach Marks is director of marketing, West Pharmaceutical Services, Inc.


